|
Research (“Interaction-based ion selectivity exhibited by self-assembled, cross-linked zwitterionic copolymer membranes”, Asatekin et al.) reported in Proceedings of the National Academy of Sciences, demonstrates novel polymer membranes can separate fluoride from chloride and other ions (electrically charged atoms) with twice the selectivity reported by other methods.
WATERTODAY spoke with lead researcher Ayse Asatekin, Associate Professor in Chemical and Biological Engineering at Tufts University, about the biology-inspired technology.
“It’s all about size. Filters can easily achieve separation between big and small. The challenge is separating same sized atoms. We have shown that we are able to create separation with special polymers that are organized at the nanometer level in a cross-linking approach that reduces pore size to under a nanometer,” Asatekin said.
“This selectivity is ideally suited for the removal of fluoride from drinking water.”
Hope For Curbing Fluoride Toxicity
Prolonged exposure to excess fluoride can cause fluorosis, a condition that can weaken the teeth, and calcify tendons and ligaments. The World Health Organization estimates that excessive fluoride concentrations have caused tens of millions of cases of dental and skeletal fluorosis worldwide.
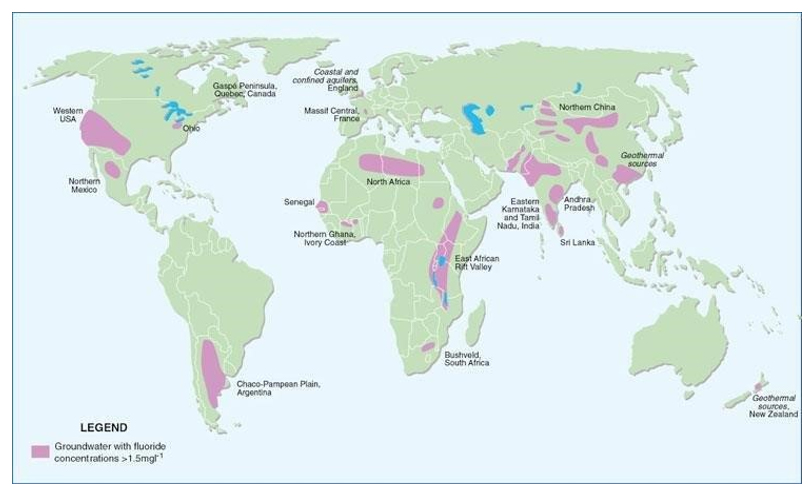
Map of documented occurrences of high or endemic fluoride in groundwater (≥1.5mb/L)
Water filtration membranes with advanced ion selectivity are urgently needed for resource recovery and the production of clean drinking water. The research out of Tufts now has shown that the application of their technology could prevent fluoride toxicity in water supplies where the element occurs naturally at too high levels for human consumption.
The Technology
“Membranes are nothing new,” Asatekin says. “They have been around for over 30 years but they have been made of the same materials that cannot do everything. With our technology, we started from scratch with a design that mimics biology. Biological ion channels (BICs) exhibit exquisite ion selectivity that can inspire novel membranes capable of precise ion separations.
“With membrane filters, you can make the pores much, much smaller. In fact, by making them 10-100 times smaller you can keep back disease-causing bacteria for instance. You can make them so small you can remove salt ions from water to produce drinking water from seawater.”
A ‘zwitterion’ (also known as an ‘inner salt’) is a molecule that has both a positively and a negatively charged group in close proximity. These charges pull water to the zwitterion while achieving selectivity.
The study found that fluoride rejection ranged between 92.5 to 88.1%, high enough to reduce the fluoride content to below the World Health Organization limit (1.5 ppm) for problematic water sources from the United States, Turkey, Germany, China, and India.
“These are places where you don’t want to remove the valuable minerals. The potential for ion-selective membranes to reduce excess fluoride in drinking water is very encouraging. The ability to remove fluoride with a relatively inexpensive filtering membrane could protect communities from fluorosis without requiring the use of high-pressure filtration or having to completely remove all components and then re-mineralize the drinking water.”
Up Next
Asatekin says the model that has been created in her lab is ready to be scaled up for commercialization.
“We’ve pretty much got the manufacturing techniques figured out,” she says. “But that’s not all. This cost-effective and environmentally friendly model will work for so many other issues, such as:
- Selective removal of contaminants from water and wastewater
- Recovery of valuable materials in water
- Off-shore drilling where traditional methods are harmful to the environment Sulfate/chloride selectivity for industrial purposes
“The driving force behind all my work is protecting our water sources.”
suzanne.f@watertoday.ca
|




